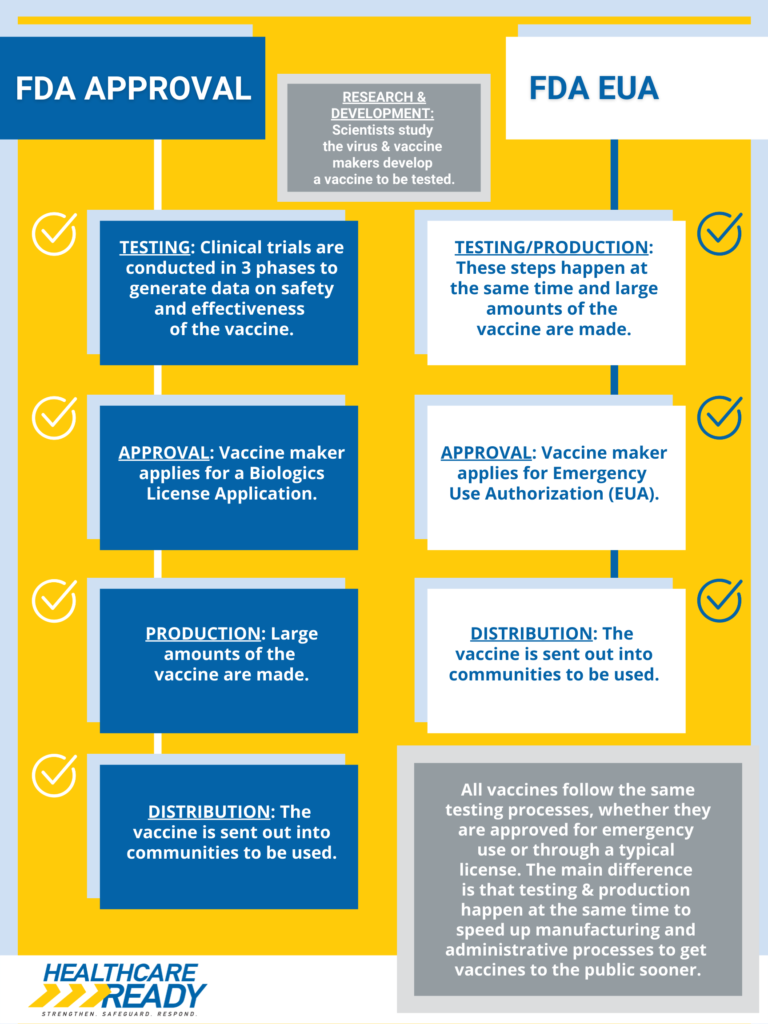
FDA Approval vs FDA Emergency Use Authorization
The FDA has officially approved the Pfizer-BioNTech COVID-19 vaccine for persons age 16 years and older. The Moderna and Johnson & Johnson/Janssen COVID-19 vaccines remain available to the public under Emergency Use Authorization (EUA). The difference between FDA approval and FDA Emergency Use Authorization can be found here.