2022 Pediatric Respiratory Surge Event #2
Healthcare Ready is ENGAGED for this event. We are monitoring potential concerns for supply chain disruptions and impacts on healthcare services.
Summary
- According to Centers for Disease Control (CDC) estimates, close to 1 out of 500 children younger than 6 months have been hospitalized with the respiratory syncytial virus (RSV) in the US since October of this year.
- RSV infections are driving a surge in pediatric emergency department visits and hospitalizations across the Nation, and are accompanied by a noted rise in other respiratory-related illnesses, such as RSV, enterovirus, and rhinovirus. Instances of co-infections between RSV, influenza, COVID-19, and bacteria are commonly observed and indicative of a possible greater surge scenario as the respiratory season progresses, which is now being referred to as a “tripledemic” (RSV, influenza, and COVID-19).
- The sensitivity of the pediatric population to RSV is adding to more severe clinical presentations, especially in infants 0-6 months old. Premature infants are especially at risk.
- The number of pediatric emergency department hospital visits and hospitalizations is nearly triple the typical values for this time of the year. As the current RSV surge shows an indication of high activity outside of its regular seasonal cycle, it is causing an early spend-down of pharmaceuticals and equipment allotted by hospitals as a part of their influenza and respiratory season preparedness activities.
- Pediatric supply chains are often more vulnerable to supply chain disruptions, as some critical products have only one supplier or manufacturer capable of producing the necessary pediatric-specific equipment and supplies.
- Since October 21, hospitals in the following states and localities have seen a rising pediatric surge: Rhode Island, Washington, Colorado, Texas, Ohio, Louisiana, New Jersey, Massachusetts, Connecticut, Maryland, Virginia, and Washington, DC, Officials from the US Centers for Disease Control and Prevention are advising hospital systems to increase communication and resource/stock-sharing to address the present surge, but there is currently no known coordinated response to this event.
Assessment of Healthcare Logistics Impacts
Pediatric Hospitalizations
According to CDC data, approximately 58,000-80,000 children under 5 were hospitalized with RSV annually in years before the COVID-19 pandemic. In typical years, RSV sends thousands of children to the hospital during the fall and winter, with a peak number of cases and hospitalizations seen in January.
During the pandemic, RSV rates flattened due to the public safety measures such as social distancing, sanitation, and masking. During the pre-pandemic years, most children would have encountered RSV within the first two years of their life. Children who did not get sick with the virus in 2020 or 2021, especially those who have not had a chance of encountering the virus yet, such as infants (0-6 months), might have less immunity and be more susceptible to more severe symptoms.
This 2022-2023 season, rates of respiratory syncytial virus (RSV) have been surging since August, which is weeks earlier than during a typical season. The RSV surge event is sending an unprecedented number of children to hospitals around the country. Doctors are warning of a “tripledemic” as cases of COVID-19, flu, and RSV are all expected to rise. An additional concern is that as winter moves people indoors, the proximity will cause an even greater increase in RSV infections.
Since August, the number of pediatric respiratory emergency department visits has almost tripled, as has the number of hospitalizations. One to two out of every 100 children younger than 6 months who contracted RSV require hospitalization. Those hospitalized children often receive critical care treatments, including oxygen support, suctioning, and IV fluids, some of which require specific pediatric equipment due to variable sizing, formulation, or dosage whose availability might be constricted during an unprecedented surge event.
A Rhode Island hospital and an additional Connecticut hospital report that within the past few weeks, the number of patients admitted with RSV has close to tripled; a Connecticut hospital in Hartford is considering creating a field hospital to help build capacity. The percentage of positive RSV cases is up 1 percent from the previous month and continues to rise in Colorado. Children’s hospitals in DC, Virginia, and Baltimore are similarly reporting that they are at the total capacity of available pediatric beds. Johns Hopkins Children’s Center in Baltimore is redeploying staff within the hospital to cope with the surge. Based on the experiences of states already impacted by pediatric respiratory illness surges, current trends indicate a likelihood that more states will be impacted.
Health Equity Concerns
Infants younger than six months, especially those who are premature, are at and especially high risk for contracting RSV and are affected by typical symptoms of this illness. Excessive medical surge caused by RSV and other respiratory diseases may threaten the health outcomes of children, especially infants if the medical surge situation worsens and crisis standards of care are implemented.
A 2013 study that investigated disparities between black and white children in hospitalizations associated with acute respiratory illness (confirmed influenza and RSV) found that Black children are hospitalized at higher rates than white children. The concern is that already underserved populations will face worse circumstances from the RSV surge. As RSV cases of babies rise to record highs, babies of color will likely experience this surge more negatively than others.
Older and elderly adults are another population more susceptible to the negative impacts of RSV. According to CDC data, 60,000 to 120,000 older adults are hospitalized with RSV, and out of those 6,000 to 10,000 die due to the infection. Adults 65 years and older and those with comorbidities, such as weakened immune systems and chronic heart and lung disease, are especially vulnerable to RSV.
Epidemiology Updates for Respiratory Illnesses of Concern
CDC maintains a Respiratory Virus Hospitalization Surveillance Network (RESP-NET), which is designed to “conduct population-based surveillance for laboratory-confirmed COVID-19, RSV, and influenza-associated hospitalizations.” RESP-NET is composed of three sets of surveillance data: Respiratory Syncytial Virus Hospitalization Surveillance Network (RSV-NET), Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET), and Influenza Hospitalization Surveillance Network (FluSurv-NET).
RSV-NET includes data by age, sex, race and ethnicity, and county of residence. The RSV-NET Interactive Dashboard shows preliminary data for 58 counties in 12 participating states (California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New Mexico, New York, Oregon, Tennessee, Michigan, and Utah) that can be used to follow trends across demographic groups.
Disclaimer: The following RSV-NET data represents only the aggregated data from participating states; trends likely differ by geography. While it does not represent “the true burden of RSV-associated hospitalizations” (which may be higher due to the high likelihood of non-laboratory confirmed RSV cases) and covers around 8% of the US population, it may be useful for extrapolating impacts to different, potentially vulnerable populations. For the areas tracked by RSV-NET:
Rates of RSV hospitalizations for children (ages 0-17) are more than three times higher than they were at the same time last year (10.3 per 100,000 children for the week ending 10/30/22, compared to 3.3 per 100,000 children for the week ending 10/30/21).
In terms of hospitalization for all ages, rates have been highest (and are increasing) for Hispanic individuals (2.3 hospitalizations per 100,000 for the week ending 10/29/22). Black, non-Hispanic individuals also have relatively high hospitalization rates (1.7 hospitalizations per 100,000 for the week ending 10/29/22), especially compared to White individuals (1.4 hospitalizations per 100,000 children for the week ending 10/29/22).
The youngest populations are experiencing the highest hospitalization rates. For the week ending 10/29, the hospitalization rate for children aged 0 to <6 months was 124.2 per 100,000. This is more than double the highest rate for the same age group for the 2021-2022 RSV season.
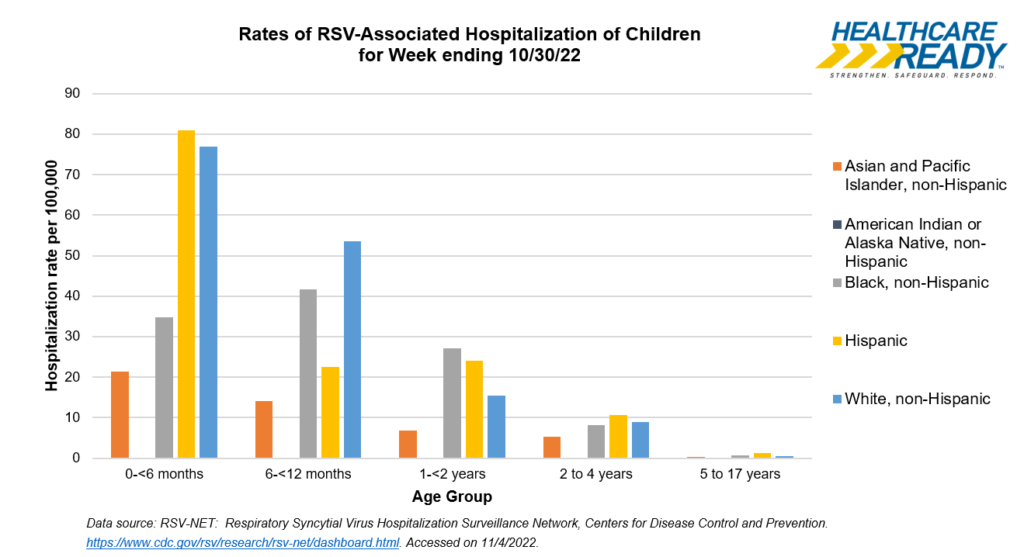
For the week ending 10/30, hospitalization rates in areas tracked by RSV-NET were highest for Hispanic children (80.9 hospitalizations per 100,000), followed by White non-Hispanic children (76.9 hospitalizations per 100,000).
CDC does not track real-time cases of, hospitalizations, or deaths from RSV throughout the country, rather, it is tracking case detection trends state by state as RSV cases rise. CDC is also tracking case trends by Department of Health and Human Services (HHS) Region and Census Region.
There are not yet detailed statistics on what percentage of patients test positive for RSV, but reports emphasize RSV has the highest positivity rates of all run tests. Doctors report that every minute or hour is critical to a child’s care. The surge in RSV cases is causing guardians to take their children hours away to find an open ER or ICU bed.
Government Response
Federal Posture
Currently, the CDC is monitoring the rise of RSV around the country and has noticed a sharp increase in RSV-related emergency department visits and hospitalizations.
The HHS Administration for Strategic Preparedness and Response (ASPR) Technical Resources, Assistance Center, and Information Exchange (TRACIE) has developed a Pediatric Surge Resources page highlighting resources to help address the response. ASPR will also be developing an email inbox to collect and answer requests for information about the surge.
In a press briefing in late October, Dr. Ashish Jha, the White House Coronavirus Response Coordinator, mentioned the high prevalence of RSV and said that the main goal is to keep hospitalizations low while the US experiences the coinciding impacts of COVID-19, influenza, and RSV. He mentioned that getting vaccinated for COVID-19 and influenza will help keep hospitalizations low as there is no vaccine for RSV yet.
At least one hospital has considered requesting Federal assets to support their response to pediatric surge. Connecticut Children’s Medical Center in Hartford reported they are working with FEMA and the National Guard to potentially set up medical tents on the hospital lawn because of the increased need for beds. If hospitalization rates continue to rise, it is likely that there will be an increase in requests for state or Federal aid.
State and Local Posture
A small number of states and local jurisdictions have instituted emergency measures to expand ability to care for children. The Oklahoma State Department of Health will temporarily allow hospitals to designate adult beds for children amid a surge in RSV cases in children statewide.
Only one jurisdiction has issued an emergency declaration for this event thus far. On October 31, the County Health Officer of Orange County, California, issued a Declaration of Health Emergency in Orange County due to rapidly spreading virus infections – especially RSV – causing record numbers of pediatric hospitalizations and daily emergency room visits. Additionally, a Proclamation of Local Emergency has been declared, which allows the County to access State and Federal resources to address the situation and seek mutual aid from surrounding counties.
If cases continue to increase, we expect additional jurisdictions to allow for emergency measures in the coming weeks.
Potential Threats for Pediatric Medical Surge
There are several challenges unique to managing pediatric medical surge, particularly for the healthcare workforce and supply chain. Pediatric hospitals require more intensive nursing resources to treat and monitor patients – especially patients in intensive care and neonatal intensive care. Pediatric supply chains can also be more vulnerable to supply chain disruptions, as some critical products have only one supplier or manufacturer capable of producing the necessary pediatric-specific equipment and supplies.
Product Availability
The oral powder form of amoxicillin, a common broad-spectrum antibiotic used to treat various bacterial infections, was recently added to the FDA drug shortage list. Other forms of amoxicillin are not on the shortages list. While amoxicillin is not a treatment for RSV (as RSV is a virus, not bacteria), children with RSV can develop symptoms that are hard to differentiate from bacterial infections. RSV can also decrease bacterial clearance, potentially predisposing them to secondary infections. These reasons are likely causing increased demand for amoxicillin. Impacts of the shortage of amoxicillin have not been reported, but the situation should be monitored.
Products of concern for this event include supplies and equipment that are commonly used to treat respiratory illnesses, and that may have limited numbers of manufacturers. Products that should be tracked for disruptions due to increased demand, include:
- Nasal cannulas (for which pediatrics has multiple sizes)
- Antivirals
- Fluids
- Pediatric ventilators
- Treatments like palivizumab
- Oxygen
- Pediatric intubation supplies
- Desitin and diapers
- Pediatric personal protective equipment (PPE), including N95s
Guidance for obtaining products and equipment that might be in shortage: organizations should first attempt to acquire them through the commercial supply chain. If the need cannot be met by the normal supply chain channels; or through another local source, such as a healthcare coalition partner or health system partner, the need should be communicated to the ESF-8 representative consistent with the established emergency management policies for that jurisdiction. For needs that cannot be met at the state or territorial level, state and territorial public health preparedness officials should consult with ASPR Regional Emergency Coordinators regarding potential federal options (such as the Strategic National Stockpile) to meet unmet health and medical needs.
Treatments for RSV
Prevention and preparedness will be essential to avoid worsening surge conditions for infants, children, and adolescents. Children and young adults – who are eligible – are encouraged to get influenza shots prior to influenza season, especially because there is yet no vaccine for RSV. This will help prevent seasonal influenza cases and lessen the risk of increased hospitalizations due to other respiratory illnesses. Fewer influenza-related hospitalizations will ensure greater bed capacity in pediatric hospitals to treat other illnesses.
A monoclonal antibody therapy called palivizumab is available as a precautionary measure to prevent severe RSV illness in certain infants and children at high risk for severe disease during the normal respiratory season. It cannot cure or treat children who are already suffering from severe cases of RSV; it is a preventative treatment. Monoclonal antibody therapy is not appropriate as it is invasive and costly. However, results seen in the delivery of monoclonal antibodies to pediatric cases were effective in mitigating symptoms. Infants born prematurely or with congenital heart or lung disease may benefit the most from this treatment. The American Academy of Pediatrics published guidance for the use of palivizumab prophylaxis to prevent hospitalizations for severe RSV during the 2022-2023 RSV Season in August 2022. Since the RSV season started earlier than anticipated, as it has the past two summers, hospitals may not be able to keep up with assuring an adequate supply of palivizumab. This would restrict their ability to give patients extra protection as they move into the respiratory virus and influenza season.
With the rising cases of RSV, some states have adjusted their guidance and policies around administration of palivizumab. The Office of MaineCare Services (OMS) supports the consideration of palivizumab in patients who would be candidates per current eligibility recommendations that can be found on their website. In California, the Department of Health Care Services is updating the policy around palivizumab by increasing the doses allowed to six doses per child.
Palivizumab is sold under the brand name Synagis, and is marketed by Sobi in the United States. Sobi purchased US rights to Synagis from AstraZeneca in 2018. We are working to determine considerations for the availability of this treatment in future assessments.
While there is not yet a vaccine for RSV, on Tuesday, November 1, Pfizer announced that they will seek FDA approval for their RSV vaccine by the end of 2022. The vaccine showed 82% effectiveness at preventing severe lower respiratory tract infections in hospitalized babies three months old and 70% effectiveness at preventing severe RSV in hospitalized infants six months old. The vaccine cut the need for infants to see a doctor because of RSV by an average of more than 50% compared with a placebo which would help keep hospitalization rates down – as there is a bed capacity issue among pediatric hospitals across the nation.
Workforce Shortages
Ongoing workforce shortages may threaten the ability for facilities to establish a predictable quality of care for patients. Because pediatrics is a specialty practice, there may be additional strain on the workforce with pediatrics experience.
Hospitals and other healthcare facilities may need to increase surveillance for respiratory illnesses among staff to reduce spread and the potential for staff being out sick. Practitioner mental health should also be considered and protected. Additional training and support for practitioners that are not used to caring for acute pediatrics cases for prolonged periods should be provided whenever possible.
Preparedness Considerations for Healthcare Facilities
There are several steps that can be taken to enhance regional preparedness. Healthcare facilities and pediatric hospitals can prepare communication and resource sharing networks to be poised for response by:
- Reviewing crisis standards of care that include pediatric strategies to avoid reaching crisis;
- Coordinating with healthcare coalitions, especially to review pediatric surge annexes;
- Training non-pediatric practitioners to be able to care for pediatric patients;
- Refreshing and reviewing transfer agreements with other children’s’ hospitals or adult hospitals with pediatric bed capacity;
- Refreshing contacts with distributors and vendors to alert them of potential needs;
- Confirming contacts with county or city public health departments in case assistance needs to be requested;
- Reviewing and restocking pediatric and NICU Critical Supply Lists;
- Following the latest guidance from key institutions, such as: American Academy of Pediatrics, CDC, NIH.
As the US healthcare system prepares for flu season this fall and winter, it will be essential that pediatric hospitals have beds available and access to the necessary medical supplies. Regional facilities should be poised to activate existing partnerships to ensure pediatric hospitals are armed with needed supplies (including via state caches, regional supplies via local healthcare coalitions, or other strategies in place to support surge or alternatives to existing supply sources). State and local public health departments may disperse information on the safety and efficacy of flu vaccines to aid in communication needs around this event, along with providing additional guidance to support clinical diagnosis or helping parents and caretakers to understand when to seek medical attention.
About Healthcare Ready
Healthcare Ready is a 501(c)(3) nonprofit organization that works to ensure patient access to healthcare in times of disaster, emergency, and disease outbreaks. We leverage unique relationships with government, nonprofit and medical supply chains to build and enhance the resiliency of communities before, during and after disasters. Learn more about Healthcare Ready
To request the help of our Emergency Operations Center, contact us at alerts@healthcareready.org.
Sign up here.to receive email notifications from Healthcare Ready