Situation Overview and Highlights
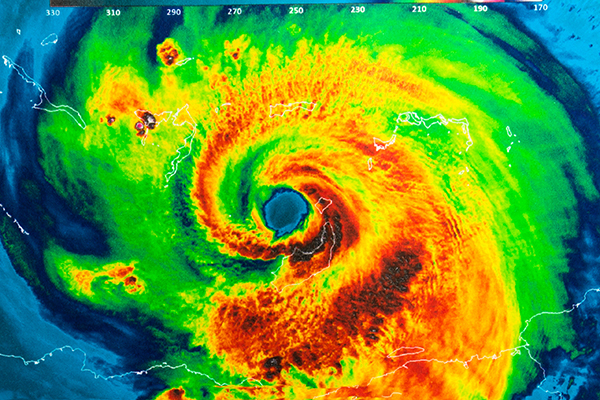
- Around noon on July 19, an EF2 tornado hit Nash and Edgecombe counties in North Carolina.
- The event caused extensive damage to a Pfizer manufacturing facility responsible for producing nearly 25 percent of all sterile injectables used in US hospitals.
- As of 4:30 pm EST, there are several reports of injuries, however, no fatalities.
- Emergency response teams are currently assessing damage, removing debris, and restoring power to the areas affected.
- Damage to the Pfizer manufacturing facility is significant given current trends in drug shortages across the nation, including several critical products, such as penicillin used to treat syphilis in pregnant women. (See our coverage on the penicillin shortage.)
- Healthcare Ready will report on downstream impacts on the healthcare supply chain as more information becomes available over the next few days.
Critical Infrastructure Impacts
- Approximately 25% of all sterile injectables utilized in US hospitals are manufactured at Pfizer’s Rocky Mount facility in North Carolina. It is one of the world’s largest sterile injectable facilities, with over 400 million units being produced annually.
- It is unclear at this time exactly which sterile injectable products are most affected by the damage.
- According to the facility website, the site produces a wide variety of products, including anesthesia, analgesia, therapeutics, anti-infectives, and neuromuscular blockers. (See Pfizer’s portfolio of sterile injectables.)
- Sterile injectables include a variety of injectable medications, including ready-to-use surgical and critical care products.
- Pfizer confirmed that at least 50,000 pallets of medicine were impacted by tornado-inflicted damage when the facility’s roof was torn off.
- The facility spans over 1.4 million square feet on a 250-acre plot in Eastern North Carolina.
Community Impacts
- A tornado, with winds of up to 135 mph, touched down in Rocky Mount, North Carolina, at about 12:30 p.m. ET on Wednesday, July 19, 2023.
- The tornado impacted the counties of Nash and Edgecombe, causing damage to residential and commercial structures, including the Pfizer facility in Rocky Mount. Some serious injuries have been reported, but no fatalities.
- A major interstate I-95 was temporarily closed with debris blocking lanes in both directions but has since re-opened.
- Emergency response teams are working to assess damage, remove debris, and restore power to the areas affected.

Related or high-value promo teaser for content here (optional)
Potential Supply Chain Impacts
- On June 12, 2023, Pfizer had released a letter to clinicians stating that there is a limited supply and impending stock out situation for select Bicillin L-A (penicillin G benzathine injectable suspension) prefilled syringes.
- This shortage had been further exacerbated as Bicillin L-A was used frequently as an alternative to treat infections in the pediatric population when amoxicillin fell into shortage.
- Pfizer had prioritized manufacturing capacity to Bicillin L-A as it is the most effective antibiotic to treat patients with syphilis, and the only product available to treat pregnant patients with syphilis.
- The infrastructure damage caused by the tornado to Pfizer’s Rocky Mount, North Carolina facility could further exacerbate the penicillin shortage because the Rocky Mount facility produced nearly 25% of all sterile injectables used in US hospitals.
- When a facility with that much manufacturing capacity goes down, the impacts to the healthcare supply chain could be considerable.
- Even if the Rocky Mount facility was not specifically manufacturing Bicillin L-A, the impact to one of Pfizer’s largest sterile injectable facilities in the world will have significant effect on the healthcare supply chain.
- In 2012, the shutdown of the PGS Rocky Mount facility (then Hospira Inc.) led to a significant impact on supply chain, causing national shortages of anesthesia and other drugs, when the FDA stepped in to rectify production issues.
Contact Us
If you become aware of situations that may adversely affect healthcare supply or patient care from this event, or if you would like to request assistance, please contact the Healthcare Ready Support Team at Alerts@HealthcareReady.org or call (866) 247-2694.